The Real Life Mind Control: Optogenetics
- Dru Norris
- Jul 25, 2025
- 2 min read
| Pheonix, Arizona
Sci-fi has long been obsessed with the idea of 'mind control'. In 2005, it was discovered that these sci-fi ideas may be more real than they seem - in the most scientific and technical context of the word - by Dr. Karl Deisseroth and Dr. Ed Boyden. While no supervillains will be controlling our every thought and action, some researchers have been able to control living cells through a genius combination of light beams and genetic engineering. This field is known as optogenetics, the use of light beams to control genetically modified cells.
Optogenetics involves inserting opsins, light-sensitive proteins, into a living cell, namely a neuron, cardiac cell, stem cell, or cancer cell to make the cell responsive to light. In the first step, the plasmid construct, which is a DNA molecule modified with injected genetic material, encoding the opsin is synthesized. While it can sound scary, this essentially means that the opsin is fused with fluorescent reporter proteins. Researchers do this because the original opsin itself is not fluorescent, and they need to be able to see their behavior in the cell. In the second step, these newly modified opsins are transfected into the cell in question. While this is done, the researcher simultaneously looks at the fluorescent reporter proteins using fluorescence imaging to visualize the process taking place.
In step three, the researchers start controlling the opsin-modified cell using light beams. They set the wavelength and pulse behavior of the light to a specific length, repetition rate, etc. to activate the opsin, thus controlling the cell. Upon successful completion, the researchers use various methods to monitor and research the cell activity according to the type of research they are doing.
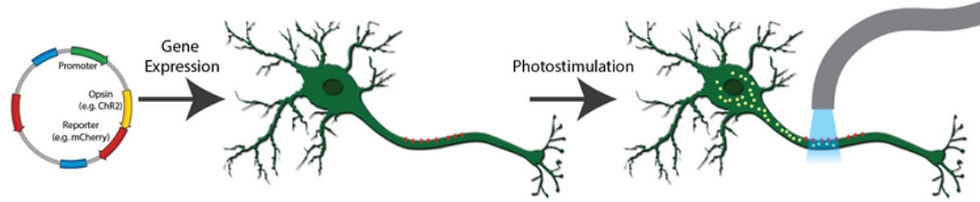
But what does the cell do when manipulated by the light beam? It depends on the type of opsin they modified the cell with. A commonly used opsin, Channelrhodopsin or ChR2, is an ion channel protein affected by blue light and is used in optogenetics to activate the cell it modifies. For example, one experiment demonstrated ChR2's ability to activate individual mechanosensory neurons in zebrafish. Another opsin, Natronomonas pharaonis halorhodopsin or NpHR, is an electrogenic chloride pump activated by yellow light. When activated, NpHR hyperpolarizes the cell, thus inhibiting it.
In practice, optogenetics has helped researchers develop strategies to control peripheral nerves, modulate cardiovascular function, breathing, and blood pressure in rats, and so much more. While we can't become sci-fi or fantasy supervillains with optogenetics, it still deserves its flowers. Even though optogenetics is relatively new, it has already made great waves in neuroscience and has a promising future.





Comments